Inflammation & Oxidative Stress
Immune/redox regulation layer — cellular protection, repair processes, inflammatory signalling tone.
Overview
Chronic inflammation and oxidative stress are treated here as one interconnected system: inflammatory and oxidative pathways reinforce each other and are targeted together by the BRAIN Diet. Chronic inflammation is a biological dysfunction that contributes to cognitive, emotional, and behavioral dysregulation in ADHD and other neurodevelopmental conditions. Neurodevelopmental disorders such as ADHD, ASD, and bipolar disorder, and neurodegenerative conditions like Alzheimer's and Parkinson's disease, share overlapping biological dysfunctions, including chronic inflammation, oxidative stress, impaired methylation, gut–brain axis disruption, glucose dysregulation, micronutrient deficiencies, and neurotransmitter imbalances. The BRAIN Diet targets inflammatory and oxidative pathways together through anti-inflammatory nutrients, antioxidant networks, gut barrier support, and strategies to reduce inflammatory and oxidative load (paper.txt, lines 132, 505). For more detail on oxidative stress mechanisms and antioxidant strategies, see Oxidative Stress.
Recipes
Therapeutic Areas
Substances
Biological Implications
Neuroinflammation Mechanisms
High levels of ROS can cause astrocytes and microglia to become activated; reactive astrocytes can release pro-inflammatory cytokines (e.g., ADHD associated biomarkers IL-6, IL-1β, TNF-α), exacerbating inflammation and neuronal damage creating a vicious cycle. This vicious circle may increase the risk of ADHD pathogenesis (paper.txt, lines 735-736).
Gut-Brain Axis and Inflammation
The gut barrier is the dynamic interface between the microbiome, immune system, and brain. Its integrity is critical: when the barrier weakens, bacterial fragments such as lipopolysaccharide (LPS, a bacterial endotoxin) and dietary antigens enter circulation, sustaining chronic low-grade inflammation and altering neurotransmitter metabolism. These processes are strongly correlated with poor cognitive function, fatigue, mood instability, and ADHD symptom severity (paper.txt, line 505).
Diets rich in probiotics, prebiotics, and fibre (which help produce short-chain fatty acids like butyrate, propionate and lactate) can enhance gut health. Furthermore, a decreased microbial diversity (alpha diversity) has also been reported in ADHD and may contribute to 'leaky gut' syndrome and contribute to the low-grade systemic inflammation reported in ADHD (paper.txt, line 521).
Butyrate and Neuroinflammation Reduction
Butyrate has anti-inflammatory effects, potentially reducing neuroinflammation associated with ADHD. Butyrate supports mitochondrial function, enhancing brain energy metabolism, which may help with cognitive impairments seen in ADHD while also aiding in reducing cholesterol and neuroinflammation (paper.txt, lines 525-526).
Propionate research suggests that increased propionate levels could help reduce neuroinflammation and enhance cognitive function while protecting the blood-brain barrier and stimulate the secretion of norepinephrine, possibly benefiting ADHD symptoms like attention and focus (paper.txt, line 527).
Omega-3 Fatty Acids and Inflammation Resolution
In a controlled endotoxemia model, high-dose EPA+DHA (3.6 g/day) attenuated fever and downstream cytokines (IL-6, IL-10), even though TNF-α remained unchanged, suggesting that omega-3s reshape the resolution phase of acute inflammation rather than block its initiation. The endotoxemia model is discussed as an analogue of postprandial inflammation, which is highly relevant as ADHD is associated with metabolic disorders, and specifically linked to postprandial inflammation (paper.txt, line 462).
Specialized pro-resolving mediators (SPMs), derived from omega-3s (DHA and EPA), play an active role in terminating inflammation without suppressing immune surveillance. These include resolvins, protectins, and maresins, which exert effects such as inhibition of neutrophil infiltration, downregulation of COX-2, and enhancement of macrophage-mediated clearance of cellular debris. Moreover, SPMs modulate endothelial function through nitric oxide release and support neuroprotection by limiting glutamate-induced excitotoxicity and promoting regulatory T-cell differentiation (paper.txt, line 460).
Postprandial Inflammation
ADHD is associated with metabolic disorders, and specifically linked to postprandial inflammation. This postprandial inflammation is relevant to the endotoxemia model, where omega-3s reshape the resolution phase of acute inflammation (paper.txt, line 462).
Immune Dysfunction and Allergies
There are many scientific findings supporting dietary antioxidant treatment of ADHD which accounted for substantial alterations in the immune system, epigenetic regulation of gene expression, and oxidative stress regulation in ADHD and also how immune dysfunction results in increased IgE levels and allergies, another link between ADHD (paper.txt, line 632).
Polyphenols and Anti-Inflammatory Effects
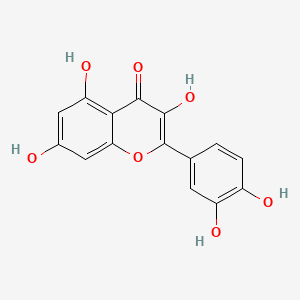
Quercetin has antioxidant, anti-inflammatory, and anti-neuroinflammatory and neuroprotective properties. Quercetin bound to a sugar molecule forming quercitrin has anti-inflammatory and anti-oxidative effects may be augmented by the co-ingestion of N-3 polyunsaturated fatty acids and olive oil (paper.txt, line 675).
Genistein, a soy-derived isoflavonoid, has shown potential as a modulator of several biochemical pathways, including the endocannabinoid system and neuroinflammation, potentially enhancing the activity of certain endocannabinoids like anandamide (paper.txt, line 677).
Carotenoids, particularly lutein, zeaxanthin, and β-carotene, play a neuroprotective role through their antioxidant and anti-inflammatory properties. These fat-soluble pigments accumulate selectively in neural tissues, including the retina and brain, where they help scavenge reactive oxygen species and stabilize cell membranes (paper.txt, line 647).
Integration with Other Systems
The BRAIN Diet targets inflammatory pathways by combining polyphenol-dense foods, omega-3s, and gut-supportive nutrients to reduce inflammatory load. Dietary polyphenols, prebiotic fibres, and lactobacilli strains have been shown to reduce LPS translocation and neutralize its inflammatory signaling, underscoring the microbiome's role in inflammation control (paper.txt, line 789).
References
- High levels of ROS cause astrocytes and microglia activation, releasing pro-inflammatory cytokines (IL-6, IL-1β, TNF-α) associated with ADHD Chang et al. 2020
- The gut barrier is the dynamic interface between the microbiome, immune system, and brain. When the barrier weakens, bacterial fragments such as lipopolysaccharide (LPS) enter circulation, sustaining chronic low-grade inflammation Mohammad and Thiemermann 2021
- A decreased microbial diversity (alpha diversity) has also been reported in ADHD Prehn-Kristensen et al. 2018
- Butyrate has anti-inflammatory effects, potentially reducing neuroinflammation associated with ADHD Yunting Li et al. 2024
- Butyrate aids in reducing cholesterol and neuroinflammation Cavaliere et al. 2022
- Increased propionate levels could help reduce neuroinflammation and enhance cognitive function while protecting the blood-brain barrier Grüter et al. 2023
- Propionate can stimulate the secretion of norepinephrine, possibly benefiting ADHD symptoms like attention and focus Hoyles et al. 2018
- In a controlled endotoxemia model, high-dose EPA+DHA (3.6 g/day) attenuated fever and downstream cytokines (IL-6, IL-10), suggesting that omega-3s reshape the resolution phase of acute inflammation rather than block its initiation Ferguson et al. 2014
- ADHD is associated with metabolic disorders, and specifically linked to postprandial inflammation Brown et al. 2025
- Specialized pro-resolving mediators (SPMs), derived from omega-3s (DHA and EPA), play an active role in terminating inflammation without suppressing immune surveillance Serhan and Petasis 2011
- Immune dysfunction results in increased IgE levels and allergies, another link between ADHD Wesselink et al. 2019
- Quercetin has antioxidant, anti-inflammatory, and anti-neuroinflammatory and neuroprotective properties Tongjaroenbuangam et al. 2011
- Quercetin bound to a sugar molecule forming quercitrin has anti-inflammatory and anti-oxidative effects that may be augmented by the co-ingestion of N-3 polyunsaturated fatty acids and olive oil Camuesco et al. 2006
- Genistein has shown potential as a modulator of several biochemical pathways, including the endocannabinoid system and neuroinflammation Fuloria et al. 2022
- Carotenoids play a neuroprotective role through their antioxidant and anti-inflammatory properties Johnson 2014