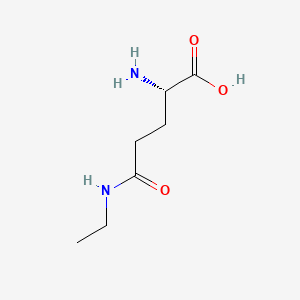
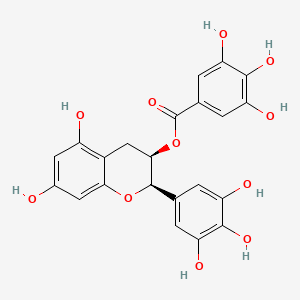
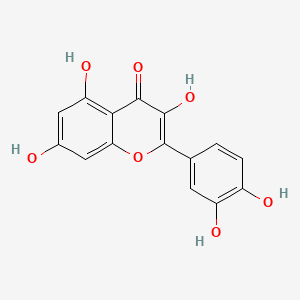
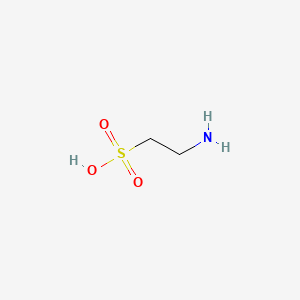
Hormonal Response
Overview
Hormonal response optimization is a critical biological target in the BRAIN Diet, focusing on balancing stress hormones, sex hormones, and other endocrine factors that significantly influence brain function, mood, and cognitive performance. The neuroendocrine and autonomic systems, together with the Enteric Nervous System (ENS), form a tightly interwoven network regulating stress responses, metabolism, and gut–brain communication. The hypothalamic–pituitary–adrenal (HPA) axis orchestrates cortisol rhythms and systemic stress signaling, while the autonomic nervous system (ANS) integrates sympathetic "fight-or-flight" activity with parasympathetic recovery pathways (paper.txt, line 769).
Recipes
Therapeutic Areas
Substances
Biological Implications
The neuroendocrine and autonomic systems, together with the Enteric Nervous System (ENS), form a tightly interwoven network regulating stress responses, metabolism, and gut–brain communication. The hypothalamic–pituitary–adrenal (HPA) axis orchestrates cortisol rhythms and systemic stress signaling, while the autonomic nervous system (ANS) integrates sympathetic "fight-or-flight" activity with parasympathetic recovery pathways. Within this circuitry, the sympatho-adreno-medullary (SAM) axis represents the most immediate arm, releasing adrenaline and noradrenaline within seconds of a stressor. These overlapping systems mediate key feedback loops between diet, inflammation, and brain function, and are highly responsive to circadian and nutritional inputs (paper.txt, line 769).
In ADHD, stress circuitry is often dysregulated. Children with sensory over-responsivity show prolonged sympathetic arousal and sustained cortisol elevations, suggesting compounded dysregulation of SAM and HPA systems. Epidemiological findings link this to clinical fatigue: while ~20% of the general population report clinically relevant fatigue, the rate in ADHD is ~62%. ADHD burnout may therefore arise from sustained sympathetic drive combined with blunted cortisol recovery, leading to chronic exhaustion (paper.txt, line 771).
Cortisol profiles in ADHD are frequently abnormal, including blunted cortisol awakening responses and flattened daily rhythms. Genetic variation in HPA-axis regulators, including NR3C1 polymorphisms, further supports a heritable component to stress dysregulation. These hormonal irregularities can invoke many ADHD related symptoms such as poor resilience, amplified impulsivity and anxiety, and impaired decision-making (paper.txt, line 774).
While epidemiological studies suggest the prevalence of metabolic syndrome in adults with ADHD (~10.8% under 60) does not exceed general population norms, there is substantial evidence of mechanistic overlap. ADHD often co-occurs with obesity, insulin resistance, and dysglycemia with shared pathophysiological pathways including dopaminergic reward circuit impairment, HPA-axis disturbances, chronic low-grade inflammation, and oxidative stress. These pathways create reciprocal vulnerability: metabolic dysfunction amplifies ADHD symptoms, and ADHD behaviors and lifestyles (poor diet, stress reactivity, irregular sleep) worsen metabolic risk and ADHD symptoms (paper.txt, line 777).
Prenatal exposures further highlight the metabolic link. Maternal diabetes is associated with significantly increased ADHD risk in offspring, with meta-analyses estimating a 44% increase overall and higher risk for pre-existing diabetes compared to gestational diabetes. Chronic maternal hyperglycemia and inflammation during neurodevelopment likely underpin this effect (paper.txt, line 778).
Many of the nutritional strategies discussed in this paper—such as glycemic stabilization, SCFA production, polyphenol intake, and anxiolytic probiotic interventions—are expected to exert downstream effects on emotional regulation through modulation of HPA axis activity, limbic signalling, and neurotransmitter systems (paper.txt, line 335).
Omega-3s (EPA/DHA) improve vagal tone and HRV control, improving cortisol rhythms and inflammation, BDNF signaling (paper.txt, line 796).
References
- The neuroendocrine and autonomic systems, together with the Enteric Nervous System (ENS), form a tightly interwoven network regulating stress responses, metabolism, and gut–brain communication Mohamed and Kobeissy 2024
- The sympatho-adreno-medullary (SAM) axis represents the most immediate arm, releasing adrenaline and noradrenaline within seconds of a stressor Wadsworth et al. 2019
- Children with sensory over-responsivity show prolonged sympathetic arousal and sustained cortisol elevations Lane 2010
- While ~20% of the general population report clinically relevant fatigue, the rate in ADHD is ~62% Rogers et al. 2017
- Cortisol profiles in ADHD are frequently abnormal, including blunted cortisol awakening responses and flattened daily rhythms Isaksson et al. 2012
- Cortisol profiles in ADHD are frequently abnormal, including blunted cortisol awakening responses and flattened daily rhythms Chang et al. 2021
- Cortisol profiles in ADHD are frequently abnormal, including blunted cortisol awakening responses and flattened daily rhythms Jue et al. 2023
- Genetic variation in HPA-axis regulators, including NR3C1 polymorphisms, further supports a heritable component to stress dysregulation Fortier et al. 2013
- Genetic variation in HPA-axis regulators, including NR3C1 polymorphisms, further supports a heritable component to stress dysregulation Carpena et al. 2022
- The prevalence of metabolic syndrome in adults with ADHD (~10.8% under 60) does not exceed general population norms Di Girolamo et al. 2022
- ADHD often co-occurs with obesity, insulin resistance, and dysglycemia with shared pathophysiological pathways including dopaminergic reward circuit impairment, HPA-axis disturbances, chronic low-grade inflammation, and oxidative stress Marcelli et al. 2025
- Omega-3s (EPA/DHA) improve vagal tone and HRV control, improving cortisol rhythms and inflammation Kiecolt-Glaser et al. 2011